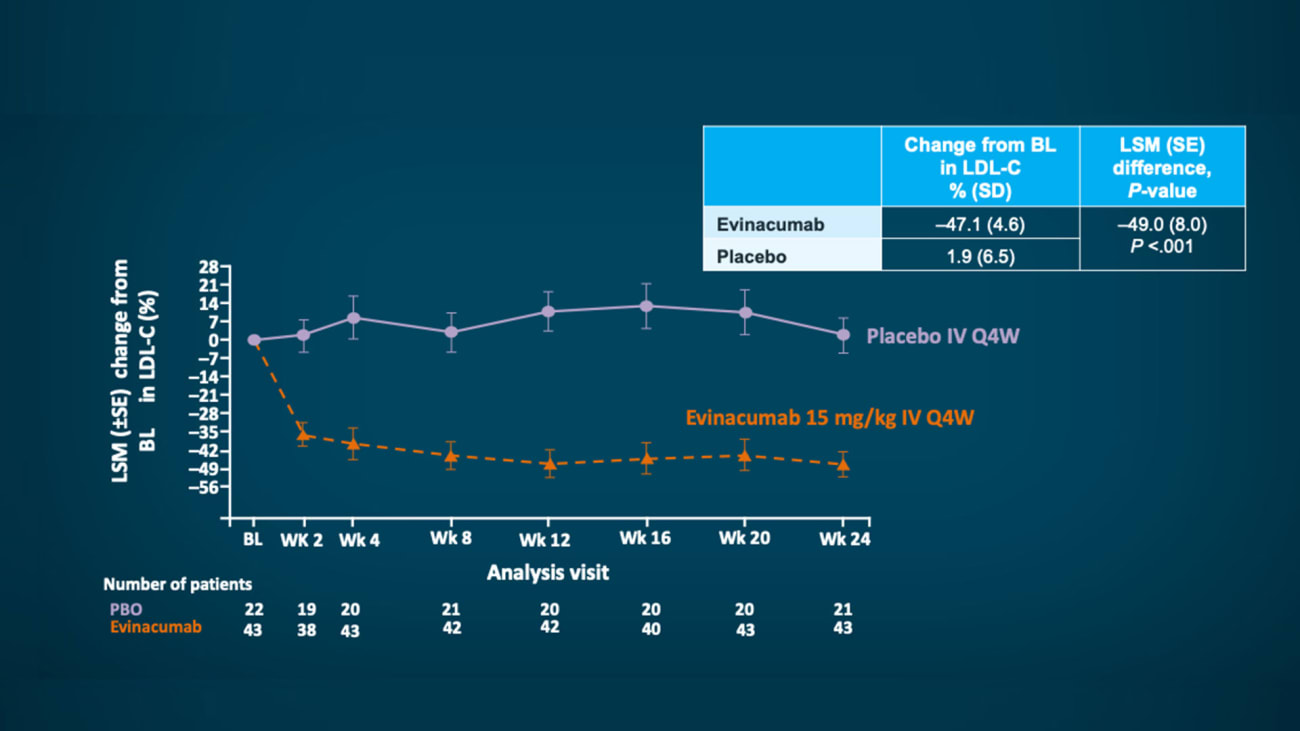
The Food and Drug Administration (FDA) in February 2021 approved evinacumab as an add-on treatment for patients aged 12 years and older with homozygous familial hypercholesterolemia (HoFH), a genetic condition that causes severely high cholesterol. The approval was based on research led by Robert Rosenson, MD, Professor of Medicine (Cardiology), Icahn School of Medicine at Mount Sinai.
The drug evinacumab reduced low-density lipoprotein (LDL) cholesterol by 47 percent in patients with severe hypercholesterolemia whose condition is resistant to standard treatments, a phase 3 study from Icahn Mount Sinai and other global academic sites found. Results from the study sponsored by Regeneron were published in August 2020 in The New England Journal of Medicine.